Overview:
This project aimed to investigate the relation between the long-range structural connectivity and neural correlates of consciousness.
Abstract:
The ability of the brain to support consciousness is determined by both the macroscale (long-range, region-to-region) and microscale (individual synapses) connectomes, as well as local information processing, which is reflected in the functional interactions between areas (functional connectome). In this project we are investigating the relation between the long-range structural connectivity and neural correlates of consciousness. We have constructed a model relating structural and functional connectivity, and we are working on simulating perturbational complexity experiments carried out in parallel in mice and plan to analyze the changes caused by perturbing the network structure. This will allow us to estimate the importance of global connection properties on neural correlates of consciousness as well as the importance of specific regions to such measures.
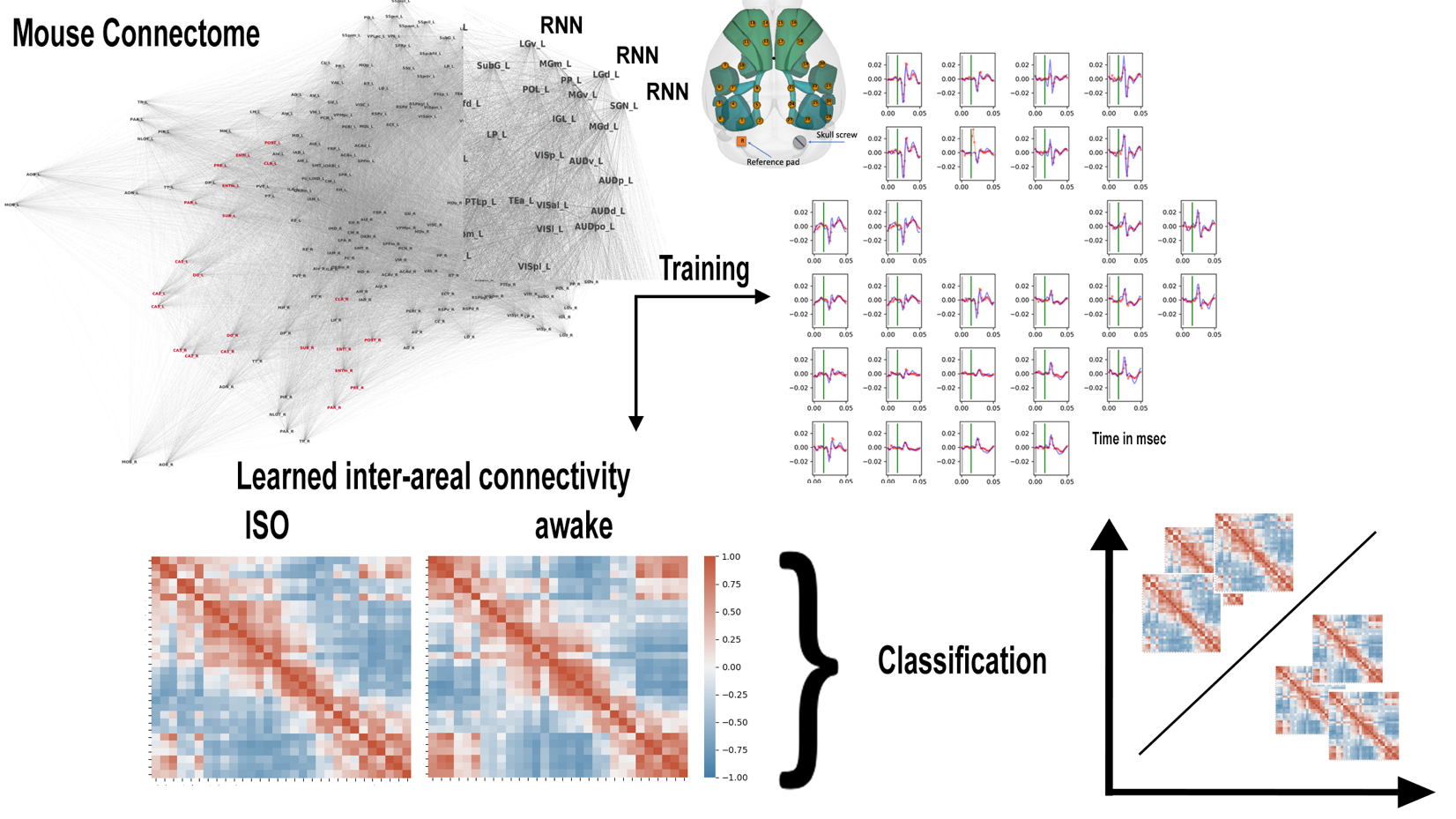
We have observed differences in evoked activity in perturbational complexity experiments, in which mice receive electrical stimulation in the deep layers of secondary motor cortex, while under isoflurane from the activity evoked in awake states. Specifically, in both conditions we observe an early wave of evoked cortical-thalamic activity, but we observe a second later wave of coordinated activity, in the both the thalamus and in cortex in awake conditions that does not occur in unconscious states. With the goal of modeling differences in inter-areal cortico-thalamic interactions that may underly these state-dependent differences in evoked activity, we have developed a multi-area recurrent neural network (RNN) model (see Figure below) that includes 198 bilateral regions (96 cortical regions, 80 thalamic regions as well as the claustrum). Each region is modeled as single layer RNN with 200 units, and all regions are recurrently connected to form a network of RNNs. The networks’ inter-regional connectivity is initialized with a biologically realistic structure derived from large-scale tract tracing from the Allen Mouse Brain Connectivity Atlas (Oh et al. Nature 2014). This connectivity will be altered over the course of training towards a representation of their functional connectivity. Training data is a sampled from a subset of stimulation trials in awake and anesthetized conditions for each mouse from their zap-n-zip EEG time series, leaving a set of trials for validation. Using this model, we have been able to reproduce EEG time series over periods of 50 msec after electrical stimulation. We train a separate model for each mouse and condition. Once trained to reproduce the EEG dynamics, the resulting models’ hidden layer parameters reveal a directed inter-areal connectivity matrix representing the underlying brain dynamics in awake and anesthetized states for each mouse. These matrices can subsequently be used to train a classifier to predict brain state. This will allow us to determine if the learned parameters are consistent across mice as well as to identify which changes in connectivity patterns are most indicative of the different brain dynamics we observe. Using artificial neural networks, we also explore the relationship between complexity, criticality, and integrated information on RNNs with different connectivity structures and explore the dynamics of their activity in learning.
Broader Impact:
With our model, we hope to distinguish differences in meso-scale whole brain functional interactions that may support conscious versus unconscious brain dynamics and the mechanisms underlying loss of consciousness associated with the administration of specific anesthetics.